Multicellular organisms engage in intricate communication between cells and organs, enabling them to adapt to fluctuating environments. Yoshizawa has focused on the complex homeostatic mechanisms mediated by organ interactions, particularly emphasizing signal transduction exemplified by the regulation of gene expression (Please see "Previous Achievements").
Since the establishment of laboratory in April 2024, we have been dedicated to elucidating novel mechanisms of intercellular and inter-organ signal transduction pathways, aiming to decode the sophisticated homeostatic maintenance systems of organisms. Our research projects encompass a variety of methodologies, including molecular biology, cell biology, biochemistry, and endocrinology, integrating these approaches to analyze processes from the microscopic molecular level to the cellular and organismal levels.
Prof. Yozahizawa Research Projects
(1) Elucidation of novel homeostatic maintenance mechanisms centered on the musculoskeletal system
Bone and skeletal muscle secrete hormones that regulate the functions of other organs, implying that musculoskeletal disorders impact not only the risk of immobility but also systemic homeostasis and lifespan. Consequently, we are advancing two primary research projects, aiming to uncover pathophysiological mechanisms beyond existing concepts and to foster the development of novel preventive and therapeutic strategies.
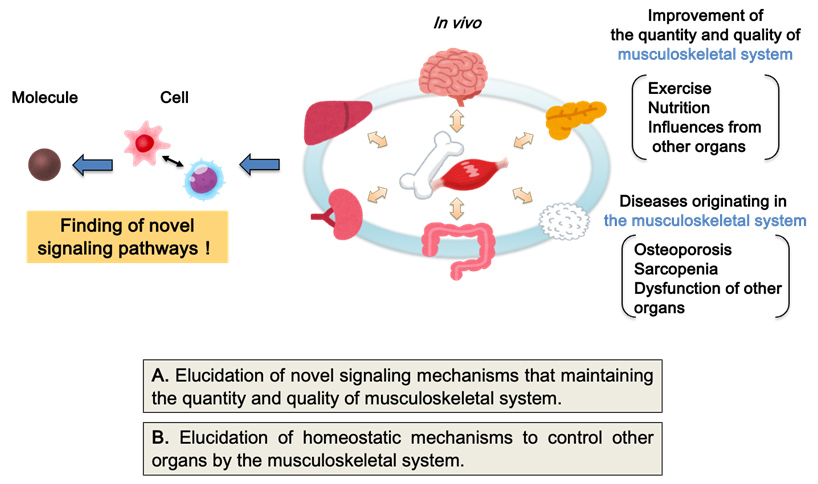
A. Elucidation of novel signaling mechanisms maintaining the quantity and quality of musculoskeletal systems
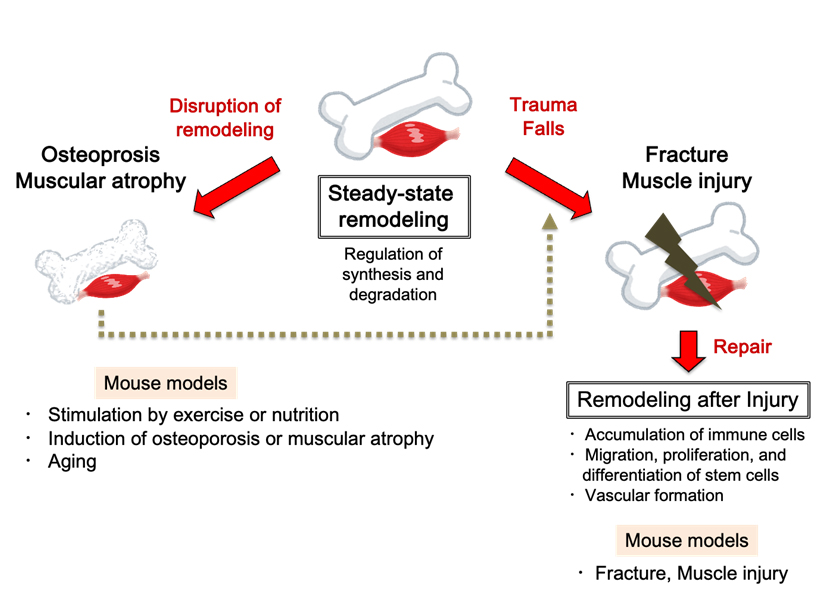
Remodeling (reconstruction: repetition of synthesis (formation) and degradation (absorption)) of bone and skeletal muscle includes steady-state remodeling and remodeling after injury such as fracture or muscle damage. In order to discover novel factors involved in bone and skeletal muscle homeostasis, we have created various mouse models of remodeling at different stages, and are conducting comprehensive gene expression and protein analysis. Currently, we are discovering new regulatory mechanisms of blood vessels and nerves in bone marrow by osteoblasts and of skeletal muscle regeneration by immune cells. We will continue to elucidate new mechanisms of musculoskeletal homeostasis that lead to healthy longevity.
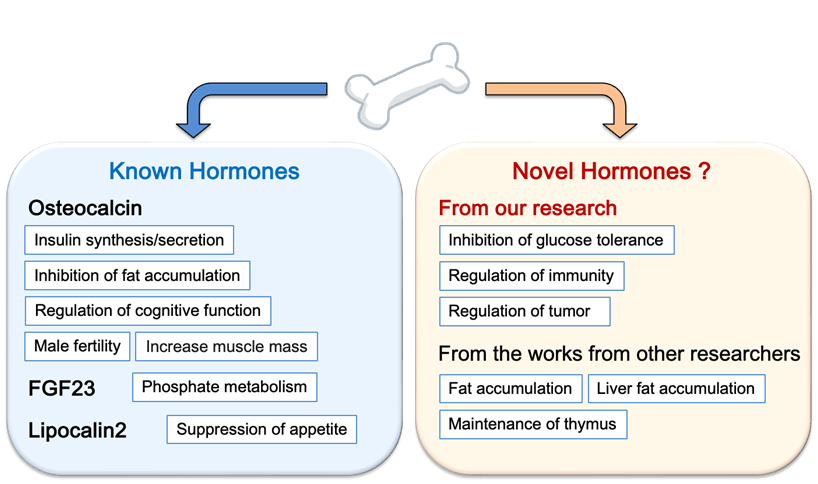
B. Elucidation of homeostatic mechanisms to control other organs by the musculoskeletal system
Recently I have discovered a novel endocrine action of bone that cannot be explained by known bone hormones (unpublished). Therefore, I am now analyzing to discover novel bone hormones and to elucidate new mechanisms of regulation of biological functions by bone tissue. Currently, we are screening candidates for novel bone hormones and analyzing them at the molecular, cellular, and individual levels.
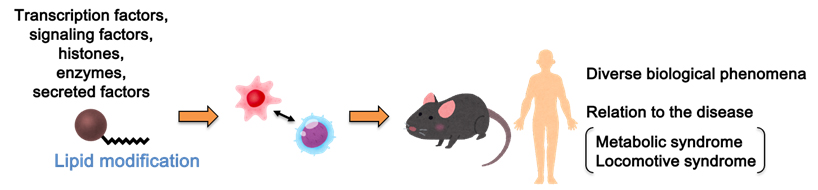
(2) Studies on the novel post-translational modifications of proteins by lipids
Lipid-mediated post-translational modification of proteins regulates protein localization, stabilization, and activity, and is deeply involved in signal transduction pathways. Therefore, we are studying the homeostatic maintenance mechanisms by fatty acylation and diseases caused by the alteration of homeostasis, and promote the construction of new concepts and research related to the basis of life phenomena.
A. Elucidation of regulatory mechanisms of nuclear hormone receptor by novel post-translational modifications
We are screening new post-translational modifications of nuclear hormone receptors and analyzing new mechanisms of functional regulation. We are currently discovering novel post-translational fatty acylation of glucocorticoid receptors involved in muscle atrophy, which we hope to use as a stepping stone to develop methods to reduce the side effects of steroid drugs.
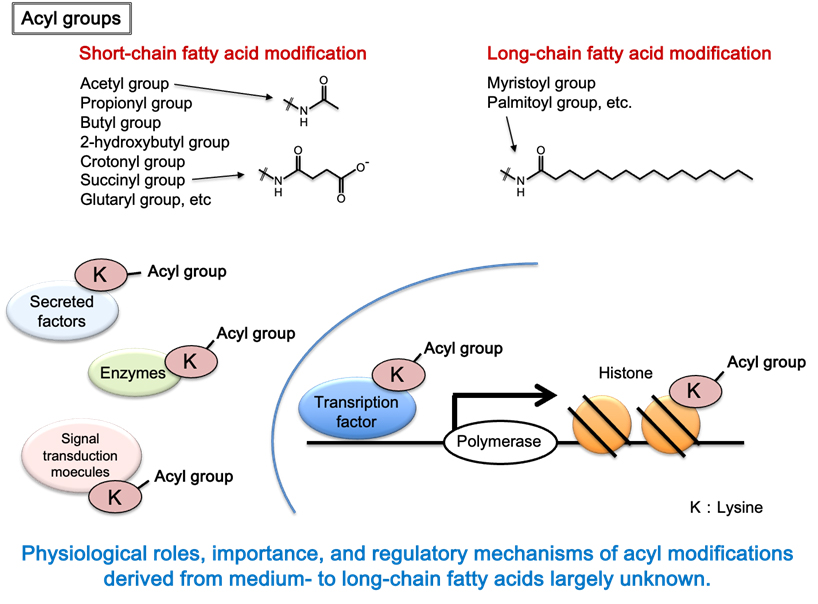
B. Study of medium-chain fatty acylation
Recently, I have discovered medium-chain fatty acylation on lysine residues, which has not been reported anywhere in the world (unpublished). There have been very few reports on medium- to long-chain fatty acylation in the world, and most of them are still poorly understood. We will continue to conduct original research to: A. elucidate the mechanism of medium-chain fatty acylation, B. identify novel medium-chain fatty acylated-proteins and analyze their functions, and C. elucidate the role of medium-chain fatty acylation in vivo.
(3) Applied Research
As part of our efforts to return our research to society, we are also conducting applied research. We are collaborating with other universities to develop a bone-directed drug delivery system as a means of delivering drugs specifically to bone. We are currently preparing to file a patent application and aim to apply and commercialize the system in the future.
Dr. Yamada Research Projects
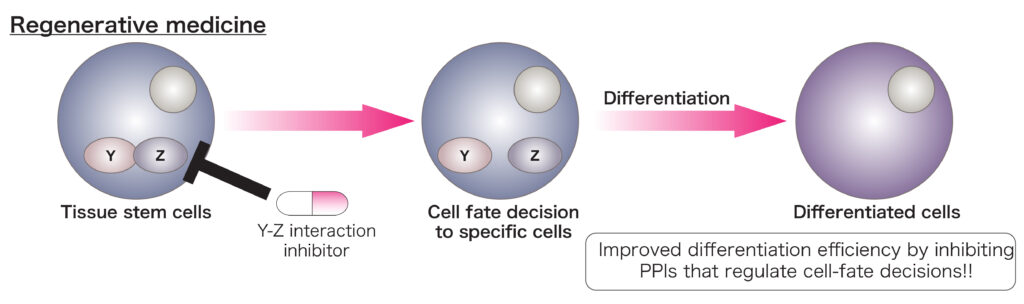
Protein-protein interactions (PPIs) have been demonstrated to regulate cell-fate decisions and tumor malignancy. In previous studies, Dr. Yamada worked on regenerative medicine and disease-modeling using human pluripotent stem cells (hPSCs) and the functional roles of PPIs in sarcoma.
In this laboratory, we have two research projects for identifing PPIs that are responsible for tumor malignancy or cell-fate decisions.
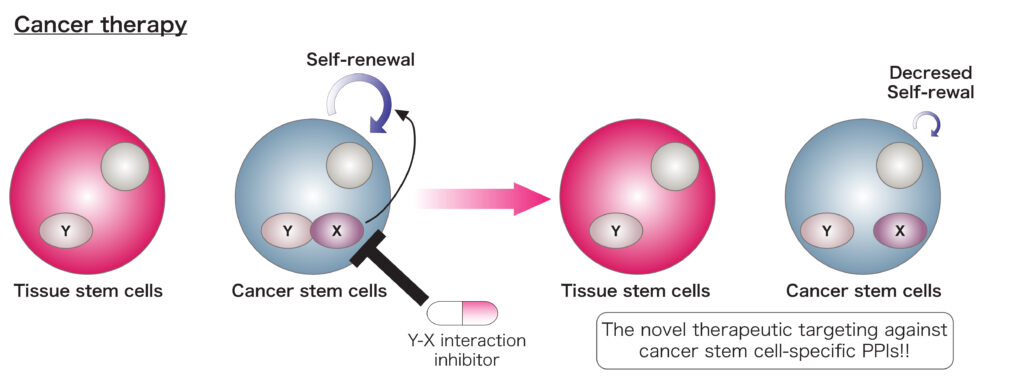
A. Regulatory roles of PPIs in tumor malignancy
Multi-lineage differentiation and limited self-renewal capacities are characteristics of tissue stem cells that plays critical roles in tissue development or regeneration. Cells with tumorigenicity, drug-resistance and self-renewal capacity in caners have been named as cancer stem cells (CSCs) or tumor-initiating cells (TICs). Although tissue stem cells and CSCs share several molecules that are essential for maintaining stem-like potential, the difference of their regulatory mechanisms have not been completely defined. Identification and inhibition of PPIs that are specific for CSCs would improve the therapeutic selectivity against CSCs and reduce the undesired effects.
B. Regulatory roles of PPIs in cell-fate decision
The methods that differentiate stem cells to desired cells with high efficiency have not been completely established. Identification and targeting of PPIs that are responsible for cell-fate decision would improve the current differentiation protocols
Dr. Gotoh Research Projects
We have studied the relationship between the development of the nervous system and intracellular metabolism. In particular, we have found that intracellular glycogen metabolism is a metabolic pathway used not only in the development of the nervous system but also as an energy source for cellular responses during inflammation. The nervous system has also been reported to project more to the skeleton and muscle, regulating their function and receiving feedback. We intend to develop research on the correlation between the skeleton and the nervous system.
A. Role of Intracellular Metabolism in the Development of the Nervous System
It is known that cellular metabolism changes during the development of the nervous system depending on the time of year and cell type. We have focused on the active glycogen metabolism in stem cells and other cells of the nervous system. We have found that the glycogen metabolic pathway is activated in mouse neonatal astrocytes and supports their proliferation (Gotoh et al., J Cereb Blood Flow Metab 2017). We have now modified genes related to glycogen metabolism by knockdown in the cerebral cortex of individual fetuses by intrauterine electroporation and analyzed their effects. We are also analyzing development using knockout mice of related genes. In this study, we are using histological and molecular biological techniques.
B. Disturbance of developmental mechanisms and long-term effects of neuroinflammation
Inflammation at specific times during development has been reported to increase the risk of autism, anxiety-like behavior, and alter seizure thresholds in adults. We are analyzing the mechanism by which LPS, a bacterial toxin, induces neuroinflammation in mice during hippocampal synaptogenesis. We found that glycogen metabolism is active in the response of astrocytes to LPS. We have shown that activation of specific energy metabolism systems is involved in the neuroinflammatory response in the central nervous system.